Insight by: Jon Warner
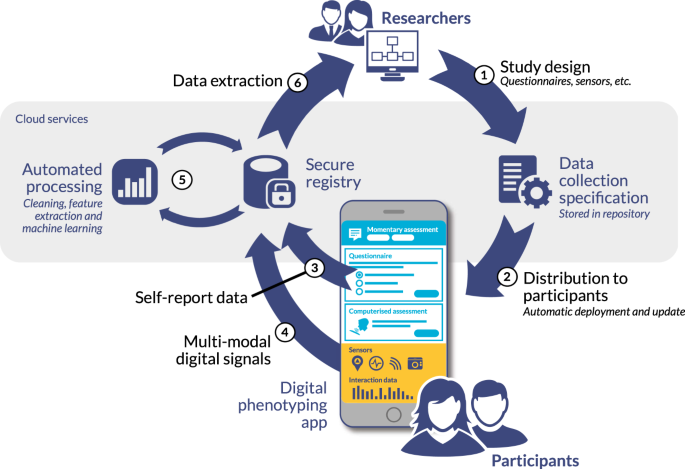
When we do research in health and healthcare, we often conduct clinical trials to ‘prove’ that we have a credible and effective solution for the target population. The best results will occur when we have a ‘balanced” population of people to study -or a range of an individual’s observable traits or phenotypes, we might then study. Digital phenotyping is relatively new process that has emerged in the last few years, that allows researchers to leverage smartphones, tablets, digital health apps, wearable data and others sources (like social media tools and platforms) to explore how technology use relates to behavioral health outcomes in particular.
Clinical trials, for pharmaceutical, medical devices and bio-technology companies have traditionally relied on periodic visits to doctors’ offices or research sites to collect data from possible participants. This can be a long and arduous process and one that could be made easier as individuals are now using technology (like smart phones and other sources as mentioned above) much more often and these can be accommodated in our thinking when looking to conduct a clinical trial. As smartphone and tablet usage continues to grow exponentially, researchers are now actively exploring new ways to leverage these powerful devices to gain a more complete picture of participants’ health and experiences during a trial. While smartphone/tablet data holds great promise to enhance clinical research, its integration also naturally brings challenges around privacy, informed consent, and ensuring a balanced representation of participants. As the use of smartphones in trials increases, developers must therefore thoughtfully address these issues to build more equitable and informative studies.
Smartphones offer an unprecedented opportunity to gather longitudinal, passive data from participants in their natural environments. Sensors and apps can continuously and unobtrusively track factors like physical activity, sleep, mood, and symptoms in near real-time. This wealth of digital phenotype data provides a more holistic view of health compared to isolated clinic visits. It also enables just-in-time feedback and interventions, which may improve outcomes. Researchers are already using smartphones to better understand conditions like depression, Parkinson’s disease, asthma and more.
However, relying primarily on smartphone data risks skewing trials toward those with access to and comfort with technology. Younger, wealthier and more educated demographics are often more likely to own smartphones and consent to research apps. This threatens to underrepresent vulnerable groups who could benefit most from new treatments. It also limits the generalizability of findings if they do not account for digital divides.
To address this, researchers must make extra efforts to engage populations who may be harder to reach digitally. Offering alternative data collection methods, providing devices to those without, and ensuring informed consent materials are accessible can help achieve a balanced sample. Multi-modal designs combining both digital and traditional clinic-based assessments also help validate smartphone findings across demographics. With care, smartphones can consequently supplement rather than replace in-person interactions, capturing a diversity of participant experiences.
Privacy and data security are also paramount concerns given the sensitivity of health and location data collected via smartphones. Participants rightfully expect strict protocols around data storage, sharing, and potential re-identification. Researchers must thoughtfully limit the types of data collected to only what is necessary for study objectives. They should also provide transparency around what data will be shared, with whom, and for how long. Obtaining ongoing consent as apps and capabilities evolve upholds respect for participants. Building trust around privacy practices now will encourage continued smartphone-based research.
Another challenge lies in developing standardized yet flexible smartphone methodologies. While consistency across trials is important, one-size-fits-all approaches risk not capturing individual variability or changes over time. Phenotyping algorithms need to account for factors like device and operating system differences, technical issues, and fluctuations in symptom severity or lifestyle. Dynamic protocols that can adapt data collection based on individual trajectories may ultimately provide richer insights. Standardized outcome measures and data formats also facilitate comparisons. Overall, smartphone methodologies must balance rigor with real-world relevance.
In summary, as clinical research increasingly seeks to leverage smartphones and tablet data, clinical trial designers and managers must thoughtfully address issues of representation, privacy and methodological rigor. Only by prioritizing diversity, security and flexibility can these digital tools enhance rather than hinder trial equity and scientific validity. But with careful planning and ongoing participant input, smart devices are already showing great potential to revolutionize how we understand and treat disease. Their integration into research promises more patient-centered, cost-effective and personalized medicine – but if only we ensure all populations can benefit by delivering more balanced and informative clinical trials.


